Background and Overview
2,4-Dibromobenzoic acid is a chemical intermediate with the molecular formula C7H4Br2O2. .
Preparation[1-3]
Method 1, CN02821215.0 discloses the two-step preparation of 2,4-dibromobenzoic acid from 2,4-dibromoaniline
The first step: 2,4-dibromobenzonitrile
At 60°C, add copper cyanide (2.32g, 25.9mmol) to stirring anhydrous dimethyl sulfoxide (50mL) to form a clear solution, then add tert-butyl nitrite (7.1mL) in one portion , 59.7mmol). To this mixture was added dropwise a solution of 2,4-dibromoaniline 21 (5.0 g, 19.9 mmol) in anhydrous dimethyl sulfoxide (30 mL) via cannula. After the addition was complete, the reaction mixture was stirred for 1 hour. After cooling to 45°C, the mixture was slowly treated with 5N hydrochloric acid (50 mL). After 5 minutes, the reaction mixture was cooled to room temperature and extracted with ethyl acetate/hexane (1:1; 2 x 300 mL). The combined organic layers were washed with water (100 mL) and brine (100 mL), dried, concentrated in vacuo, and purified by silica chromatography (0-5% ethyl acetate in hexane) to afford the title compound (1.61g, 31% yield). FD(+)MS m/z 259, (M+) consistent with 2 Br.
Step 2: 2,4-dibromobenzoic acid
A stirred suspension of 2,4-dibromobenzonitrile (1.57g, 6.0mmol) in sulfuric acid (6M, 150mL) was heated to reflux for 3 days. The reaction mixture was cooled to room temperature and extracted with ethyl acetate (2 x 75 mL). The combined organic layers were washed with water (100 mL) and brine (50 mL), dried, concentrated, and then purified by silica chromatography (acetic acid/methanol/chloroform, 0.1:0.5:99.4) to obtain the title compound (0.81 g, Yield 48%). mp 171-172℃; ES(–)MS m/z 277, (M-H)– consistent with 2 Br.
Method 2
Tashiro et al. reported that 2,3-dibromobenzaldehyde was oxidized with potassium permanganate to prepare 2,4-dibromobenzoic acid, with a yield of 97%.
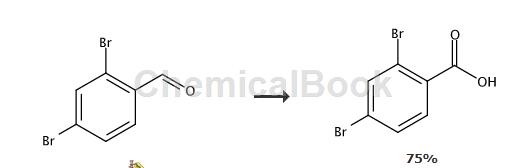
Method 3
Corbett et al. reported that 2,3-dibromoiodobenzene was reacted with butyllithium and carbon dioxide at -100°C to prepare 2,4-dibromobenzoic acid, with a yield of 91%.
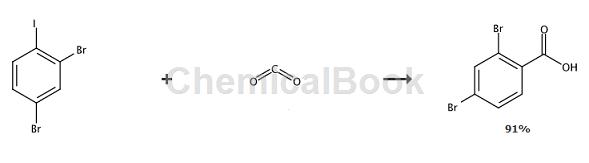
Main reference materials
[1] CN02821215.0 Thiophene and thiazolesulfonamide compounds used as antitumor agents
[2] Tashiro, Masashi and Nakayama, Kouji,From Organic Preparations and Procedures International, 16(5), 379-83; 1984
[3] Heiss, Christophe et al.From European Journal of Organic Chemistry, (23), 4625-4629; 2003

 微信扫一扫打赏
微信扫一扫打赏

