Background and overview[1][2]
4-Fluoroanisole is a colorless to slightly yellow liquid. It is an important intermediate for medicines, pesticides, and liquid crystal materials and is widely used.
Apply[3-5]
4-Fluoroanisole is an important pharmaceutical intermediate product, and its market demand, especially in Europe, America and Southeast Asia, is increasing year by year. Examples of its application are as follows:
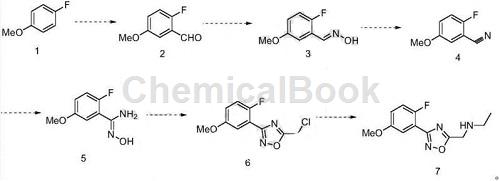
1. Preparation of N-((3-(2-fluoro-5-methoxyphenyl)-1,2,4-oxadiazol-5-yl)methyl)ethylamine and related derivatives
N-((3-(2-Fluoro-5-methoxyphenyl)-1,2,4-oxadiazol-5-yl)methyl)ethylamine and related derivatives in medicinal chemistry and has wide applications in organic synthesis. At present, the synthesis of N-((3-(2-fluoro-5-methoxyphenyl)-1,2,4-oxadiazol-5-yl)methyl)ethylamine is relatively difficult. Therefore, it is necessary to develop a synthesis method with easily available raw materials, convenient operation, easy control of the reaction, and suitable overall yield. Research has developed a fluorine-containing benzene-substituted oxadiazole compound N-((3-(2-fluoro-5-methoxyphenyl)-1,2, 4-oxadiazole-5-yl)methyl ) The preparation method of ethylamine uses 4-fluoroanisole as the starting material, and obtains the target product through aldehyde, oximation, elimination, addition, ring closure, and substitution reactions.
2. Preparation of p-fluorophenol
Fluorophenol (4-Fluorophenol) is white crystal and corrosive. Soluble in water and easily soluble in organic solvents such as ethanol and ethyl acetate. P-fluorophenol is mainly used in the synthesis of the antihypertensive drug nebivolol, 5-HT1A receptor antagonists and anti-inflammatory, anti-platelet aggregation, antibacterial and other drugs; it is also used in the synthesis of the highly effective herbicide flufenac; it can also be used Used in the production of liquid crystal materials. The preparation method is as follows: 4-fluoroanisole and hydrobromic acid undergo a demethylation reaction to generate p-fluorophenol and methyl bromide. The synthesized p-fluorophenol is separated through the extraction method and reaction system; the raffinate is passed into hydrogen bromide and recovered for use. The methyl bromide generated by the demethylation reaction is directly passed into an aqueous solution containing 15% sodium p-toluene sulfinate to generate p-methyl sulfone; the mother liquor after separating p-methyl methyl sulfone is concentrated to obtain bromide sodium. The sodium bromide obtained can be used in the step of generating hydrogen bromide. In this way, the discharge of acidic wastewater and by-products is fundamentally eliminated, and the requirements for clean production are met.
3. Synthesis of aldose reductase inhibitor fidarestat
The method is to use 4-fluoroanisole as the starting material, and obtain the target through Friedel-Crafts-acylation substitution, cyclization, acyl chlorination, resolution, hydrolysis, hydantoinization, alcohol esterification, and ammonolysis. Compound Fidarestat.
Results: The chemical structure of the target compound was confirmed by infrared spectrum, hydrogen nuclear magnetic resonance spectrum, and mass spectrum. The total yield was 14.5%.
Conclusion: When preparing intermediates 2 and 7, the process was improved and the yield was increased.
Preparation [1-2]
Method 1: A preparation method of 4-fluoroanisole, including the following steps:
① Generation of 4-fluoroanisole: In the reactor, add p-bromofluorobenzene, solvent DMF, methanol solution of sodium methoxide and catalyst in sequence, and heat the above reaction system while stirring to generate 4-fluorobenzole. For the reaction of ether, the reaction continues during the heating process, and the reaction lasts for 10 to 16 hours. After the reaction is completed, the reacted mixture is filtered to remove the catalyst to obtain a filtered liquid mixture; the catalyst is cuprous chloride or bromine. Copper.
② Separation of 4-fluoroanisole and DMF: Add the liquid mixture material obtained in step ① to the distillation device, and then add water or steam as an entrainer to make the liquid mixture material become a water-added liquid material. The water-added liquid material system is then subjected to azeotropic distillation. 4-fluoroanisole and water are azeotropically evaporated, and DMF will not azeotrope with water. For the liquid object obtained by azeotropic distillation, follow the method of separating the organic phase and the inorganic phase. Separation was performed to obtain an organic phase.
③ Distillation and purification: Send the organic phase obtained in step ② to the distillation device for distillation, and collect the main fraction to obtain the finished product of 4-fluoroanisole.
4-Fluoroanisole is prepared by direct etherification of p-bromofluorobenzene (a brominated aromatic hydrocarbon). The conversion rate of p-bromofluorobenzene reaches 99.9%. Selection of converting p-bromofluorobenzene into 4-fluoroanisole The purity is as high as over 95%, so the yield of 4-fluoroanisole is relatively high, and the purity of the finished product can reach 99.7%.�Since the conversion rate of p-bromofluorobenzene has reached more than 99.9%, in the subsequent purification operation, the separation problem of unreacted p-bromofluorobenzene and the product 4-fluoroanisole can be avoided, and the remaining relatively A small amount of p-bromofluorobenzene remains in the product 4-fluoroanisole without affecting the quality of the product. Using water or steam as an azeotropic agent, the azeotropic distillation method is used to simply separate DMF and 4-fluoroanisole, which have similar solubility and volatility, which not only ensures the yield of 4-fluoroanisole, but also This method also makes this method suitable for industrial large-scale production.
Method 2: A new preparation method of 4-fluoroanisole, which has low production cost, greatly reduces environmental pollution, and has strong market competitiveness. The technical solution is to use fluorobenzenes as starting materials and directly perform etherification reaction in an alkaline system in the presence of polar solvents and metal catalysts to obtain 4-fluoroanisole. The improvement is that the fluorobenzenes used are The molar ratio of halogen-containing fluorobenzene to alkali and methanol raw materials is halogen-containing fluorobenzene: alkali: methanol = 1:2~ 4:2~5, the polar solvents used are chloroform, nitromethane, and N’N-dimethylformamide. Any one or a mixture thereof, the usage amount of the polar solvent is 10% to 50% of the total weight of the materials, the metal catalyst is an oxide or compound of copper or zinc, and the reaction temperature of the preparation method is 60 to 95℃, the reaction time is 3 to 6 hours. A further solution is: the halogen-containing fluorobenzene is p-bromofluorobenzene or p-chlorofluorobenzene; the raw material base used is NaoH or KOH; the metal catalyst used is CuO or Cu2O.

Main reference materials
[1] CN201110054884.5 Preparation method of 4-fluoroanisole
[2] CN200710025964.1 Preparation method of 4-fluoroanisole
[3] CN201610755263.2 Preparation method of fluorine-containing benzene-substituted oxadiazole compounds
[4] CN201810448002.5 Preparation method of p-fluorophenol
[5] Cao Ruiqiang, Wang Yanxiang, He Weiying, et al. Synthesis of aldose reductase inhibitor fidarestat [J]. Chinese Journal of New Drugs, 2006, 15(6): 451-454.

 微信扫一扫打赏
微信扫一扫打赏

