Background and overview[1][2]
2-Bromo-4-fluoroacetophenone CAS number 1006-39-9, chemical formula C8H6BrFO. Molecular weight 217.03500. Melting point 48-50°C, boiling point 150°C 12mm, flash point 110°C, refractive index 1.549, vapor pressure 0.0138mmHg at 25°C. 2-Bromo-4-fluoroacetophenone can be used as an intermediate for pharmaceutical and chemical synthesis. If 2-bromo-4-fluoroacetophenone is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1-3]
2-Bromo-4-fluoroacetophenone can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its application are as follows:
1. Synthesis of new 2-benzoylbenzofuran analogues. Benzofuran compounds are widely present in a variety of medicinal plants, and their good physiological activities have attracted the attention of domestic and foreign scholars. For example, Ailanthoidol has anti-tumor and anti-viral activities, while Morin A-H, Stemofurans A-K and Nomonol have good anti-bacterial and anti-fungal activities. The reaction process is as follows:
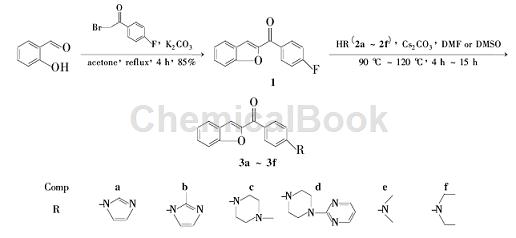
The synthesis of intermediate 1 uses 2-bromo-4-fluoroacetophenone as one of the raw materials. The reaction steps are as follows: add salicylaldehyde 2.44 g (20mmol) and K2CO3 5.52 g (40 mmol) and 60 mL of acetone, add 4.34 g (20 mmol) of 2-bromo-4-fluoroacetophenone in batches at room temperature with stirring, complete the addition, and reflux for 4 hours (TLC detection). Cool to room temperature, concentrate in vacuo, add 80 mL of water to the residue, and stir for 20 min. Suction filtration, the filter cake was washed with 10% KOH solution (20 mL) and water (3 × 20 mL) in sequence, and dried to obtain 14.08 g of yellow-brown solid, yield 85%, m.p. 188.4 ℃ ~ 190.6 ℃.
2. Synthesis of N-heterocyclic substituted benzofuran derivatives. Benzofuran compounds are widely present in a variety of medicinal plants and have attracted much attention because of their good physiological activities, such as anti-tumor, antiviral, antibacterial and antifungal activities. Benzofuran compounds are widely present in a variety of medicinal plants. Among used plants, its good physiological activity has attracted much attention from scholars at home and abroad. For example, Ailanthoidol has anti-tumor and anti-viral activities, while Morin A-H, Stemofurans A-K and Nomonol have good anti-bacterial and anti-fungal activities. The reaction process is as follows:
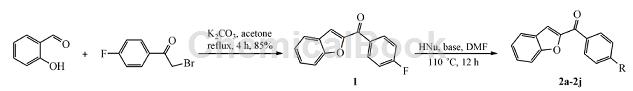
Step 1: Synthesis of intermediate 2-(4′-fluorobenzoyl)benzofuran (1)
Weigh 2.44 g (20 mmol) of salicylaldehyde and 5.52 g (40 mmol) of K2CO3 into a 100 mL round-bottomed flask, add 60 mL of acetone, and add 2-bromo-4′-fluorobenzene in batches while stirring at room temperature. 4.34 g (20 mmol) of ethanol, heated to reflux for 4 h. After TLC detection, the reaction was completed, cooled to room temperature, and concentrated in vacuo. Add 80 mL of water to the reaction flask, stir for 20 min, and filter with suction. The solid is washed with 20 mL of 10% KOH solution and water (20 mL × 3), and dried to obtain 4.08 g of a yellow-brown solid with a yield of 85%. mp: 188.4 ~ 190.6 ℃.
Step 2: Synthesis method of target compounds 2a ~ 2c
Weigh 1.20 g (5 mmol) of compound 1 and 3.26 g (10 mmol) of Cs2CO3 into a 50 mL round-bottomed flask, add 20 mL of anhydrous DMF to dissolve, then add 10 mmol of imidazole, and place at 110°C for reaction 12h. After TLC detection of reaction completion, cool to room temperature, pour the reaction into 50 mL of water, and stir for 20 min. Filter with suction, wash the solid with water (20 mL × 3), dry it, and perform column chromatography using dichloromethane-methanol (50: 1 ~ 30: 1) as the eluent.
3. Synthesis of new 4′-(N-substituted-1-piperazinyl) chalcone derivatives. Chalcones are a class of compounds with diaryl-substituted α, β-unsaturated ketones as the basic skeleton, and are widely distributed in nature. Chalcones have a unique molecular structure, contain multiple reaction centers, and can bind to a variety of receptors, thereby exhibiting a wide range of biological activities. They are a very important class of organic synthesis and drug synthesis. Chalcones extracted and isolated from natural products and synthesized through chemical, biological and other methods exhibit various pharmacological properties such as anti-tumor, anti-parasitic, anti-viral, anti-bacterial, anti-inflammatory, and anti-platelet aggregation. Therefore, the research and development of chalcones has become a hot research field in medicinal chemistry. Starting from p-dimethylaminobenzaldehyde and 2-bromo-4-fluoroacetophenone as raw materials, 4-dimethylamino-4′-(1-piperazinyl)char is generated through aldol condensation, dehydration and substitution reaction. After ketone (2), and then through substitution reaction, 10 unreported chalcone derivatives containing substituted piperazine were designed and synthesized. The bromide thiazolyl blue tetrazolium (MTT) method was used The in vitro anti-tumor activity of the compounds was preliminarily tested (human cervical cancer cell Hela, human lung cancer cell A549 and human gastric cancer cell SGC7901). The results showed that some compounds showed good inhibitory activity against tumor cell lines. For Hela, the IC50 values of A549 and GC7901 are 16.45, 0.19 and 0.78 μmol/L respectively, which needs further study.
Main reference materials
[1] Mao Zewei, Guo Wenlian, Jiang Yuan, et al. Synthesis of new 2-benzoylbenzofuran analogues[J]. Synthetic Chemistry, 2014, 22(6): 785-788.
[2] Mao Zewei, Wan Chunping, Jiang Yuan, et al. Synthesis and antitumor activity of N-heterocyclic substituted benzofuran derivatives[J]. Chinese MedicineJournal of China University of Science and Technology, 2015, 46(001): 58-61.
[3] Lin Yuping, Hu Chunyan, Zheng Xi, et al. Synthesis and anti-tumor activity of new 4′-(N-substituted-1-piperazinyl) chalcone derivatives[J]. Chin. J. Org. Chem, 2017, 37: 237-241.

 微信扫一扫打赏
微信扫一扫打赏

