Background and overview[1]
2,4-Dichloro-5-methoxyaniline CAS number 98446-49-2, chemical formula C7H7Cl2NO. Molecular weight 247.06100. White or off-white powder, density 1.375 g/cm3, melting point 51 °C, boiling point 290.1ºC at 760 mmHg, flash point 129.3ºC, refractive index 1.587. 2,4-Dichloro-5-methoxyaniline is mainly used in the preparation of primary Sutinib intermediate 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile . Bosutinib, developed by Wyeth Pharmaceuticals in the United States, was approved by the European Union as an “orphan drug” in September 2010 for the treatment of chronic myelogenous leukemia (CML). On September 4, 2012, the drug was approved by the U.S. Food and Drug Administration (FDA) for marketing under the trade name Bosulif. It was approved for marketing by the European Food and Drug Administration (EMA) on March 27, 2013, and approved by Japan’s PMDA on September 26, 2014. Studies have disclosed that the condensation reaction of ortho-formate aniline derivatives and nitrile acetaldehyde diethyl acetal produces imine derivatives, and the intermediate is cyclized under alkaline conditions to form 4-oxo-3-quinolinemethane. Nitrile derivatives. This method is one of the current mainstream preparation methods, but the yield is low, especially for the preparation of the key intermediate 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy- The yield of 7-(3-chloropropoxy)-3-quinolinecarbonitrile (formula III) is only about 50%.
Apply[1]
2,4-Dichloro-5-methoxyaniline is mainly used in the preparation of bosutinib intermediate 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6- Methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile. Some experiments have provided a simple and simple preparation method for bosutinib. This method effectively increases the yield by improving the reaction conditions, and is more suitable for industrial production than the existing technology. The specific technical solutions are as follows:
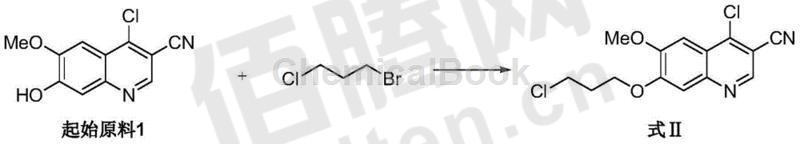
1) Using 4-chloro-6-methoxy-7-hydroxy-3-quinolinecarbonitrile as the starting material 1, in a specific aprotic polar solvent, using crown ether as the catalyst and containing potassium ions The base acts as an acid binding agent and reacts with 1-bromo-3-chloropropane for alkylation to produce 4-chloro-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile (Formula II ). The molar ratio of starting material 1 to 1-bromo-3-chloropropane is preferably 1:4 to 1:1, most preferably 1:3; the reaction temperature is preferably 20°C-60°C; the reaction is preferably carried out under nitrogen protection The specific aprotic polar solvent is selected from 1-methyl-2-pyrrolidone, dimethylacetamide and dimethylformamide, preferably 1-methyl-2-pyrrolidone; the catalyst is selected from 18-crown- 6 ether, dibenzo-18-crown-6 ether and diazo-18-crown-6 ether, preferably dibenzo-18-crown-6-ether; the acid binding agent is preferably potassium hydroxide and potassium bicarbonate and potassium carbonate, most preferably potassium carbonate.
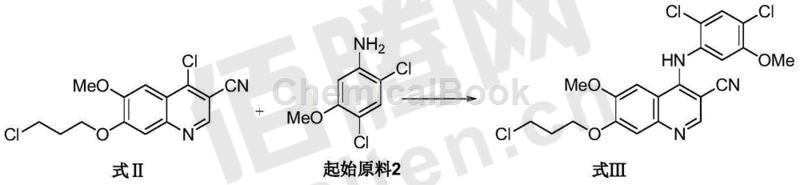
2) In the presence of pyridine hydrochloride, 4-chloro-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile (formula II) and 2,4-di Chloro-5-methoxyaniline (starting source 2) undergoes a condensation reaction in a specific aprotic polar solvent to obtain 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6- Methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile (Formula III). The molar ratio of the compound of formula II to 2,4-dichloro-5-methoxyaniline is preferably 1:1 to 1:4, most preferably 2:3; the reaction temperature is preferably 60°C-120°C, most preferably 80℃-110℃; the reaction is preferably carried out under nitrogen protection; the specific aprotic polar solvent is selected from 1-methyl-2-pyrrolidone, dimethylacetamide and dimethylformamide, preferably 1-methyl Base-2-pyrrolidone;
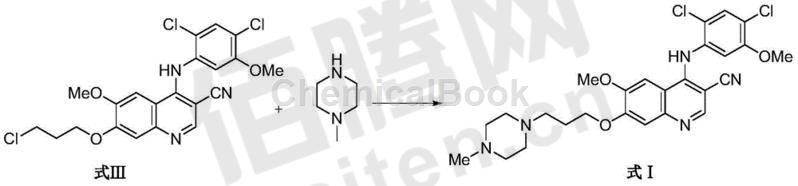
3) Using iodide as a catalyst, 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-(3-chloropropoxy)- An alkylation reaction occurs between 3-quinolinecarbonitrile (Formula III) and N-methylpiperazine in a specific aprotic polar solvent to obtain bosutinib (Formula I). The molar ratio of the compound of formula III to N-methylpiperazine is 1:2 to 1:8, most preferably 1:3-1:4; the reaction temperature is preferably 60°C-120°C, most preferably 80°C-110°C ℃; the reaction is preferably carried out under nitrogen protection; the catalyst is selected from potassium iodide, sodium iodide, magnesium iodide and lithium iodide, preferably sodium iodide; the specific aprotic polar solvent is selected from 1-methyl-2- Pyrrolidone, dimethylacetamide and dimethylformamide, preferably 1-methyl-2-pyrrolidone.
Main reference materials
[1] CN201410546745.8 A preparation method of bosutinib

 微信扫一扫打赏
微信扫一扫打赏

