Background and overview[1][2]
Sunlight is an indispensable substance for the survival of all things in the world. Appropriate ultraviolet radiation is helpful to human health and enhances human physique. However, if the skin is exposed to the sun for a long time, the medium-wave ultraviolet rays of 280~320 nm will And long-wave ultraviolet rays of 320~400 nm can cause harm to the human body. Therefore, in order to protect the human body from the harm of ultraviolet radiation, we use ultraviolet absorbers that are safe and efficient in absorbing ultraviolet rays and are widely used in textiles, coatings, sunscreens, cosmetics and other products.
There are many types of ultraviolet absorbers, among which benzophenone ultraviolet absorbers are widely used and have great practical research value. Avobenzone [chemical name: 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione] belongs to the benzophenone UV absorber and is also A very important intermediate in organic synthesis.
At present, the main methods for synthesizing avobenzone are: ①Condensation addition elimination hydration method, that is, p-methoxyacetophenone and p-tert-butylbenzaldehyde undergo condensation, bromine addition, elimination to form alkyne, and hydration The product is obtained with a yield of 58%. This method has a long route, high cost and low yield; ② Claisen ester condensation method, that is, the reaction of p-methoxyacetophenone and p-tert-butyl methyl benzoate, the yield is 63.6%, this method is low cost and easy to operate, but the yield is low; ③Aldehyde-ketone condensation method, that is, p-methoxyacetophenone and p-tert-butylbenzaldehyde are condensed into alcohol and oxidized to obtain it, with a yield of 34.6 %, this method is easy to operate, has high cost and low output.
Application
Avobezone is a synthetic ultraviolet absorber. It is a good UV-A (>320nm) type ultraviolet absorber. It can block UVA in the whole band (320~400nm) and is highly efficient. The wide spectrum oil-soluble UVA filter, when combined with other UVB sunscreens, can provide full UVA and UVB protection for the prevention of photoinduced skin cancer.
The study found that avobenzone can significantly disrupt the interaction between the two proteins PHB and c-Raf1, and for the first time confirmed through molecular biology experiments and cytology experiments that avobenzone can significantly inhibit the growth of colon cancer cells. The transfer expands the application scope of avobenzone and improves its application value. Compared with traditional chemotherapy drugs, avobenzone has high specificity in targeting tumors, has fewer side effects and clearer countermeasures to side effects.
Compared with existing tumor-targeted drugs, avobenzone does not target a single gene or protein, but the interaction between two proteins. It will not have a sustained effect on specific molecules or single proteins in cancer cells. Drug pressure leads to mutations, which greatly reduces the emergence of clinical resistance.
Preparation[1,3-4]
Method 1: Use highly active p-tert-butylbenzoyl chloride instead of p-tert-butylbenzoyl methyl ester, choose sodium amide as base, the mole ratio of p-methoxyacetophenone and p-tert-butylbenzoyl chloride The ratio is 0.85, and the condensation reaction is carried out at a constant temperature of 90°C for 5 hours to generate avobenzone, and the yield can reach 81.5%. This method has short route, short reaction time, low cost and high yield, and is an ideal process for realizing industrial production.
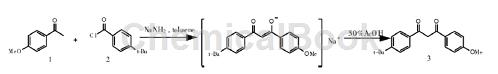
The specific steps are as follows: Dissolve 7.50 g (0.05 mol) p-methoxyacetophenone in 100 mL anhydrous toluene in a 250 mL three-necked flask under nitrogen protection, and then add 2.15 g (0.055 mol) sodium amide. Heat and constant temperature to 40 ℃, drop 12.20 g (0.0575 mol) p-tert-butylbenzoyl chloride within 30 minutes, raise the temperature to 90 ℃, react at a constant temperature for 5 hours, a large amount of solid will be generated, and cool to room temperature.
Add 50% acetic acid under constant stirring to make the entire system weakly acidic (pH = 5~6), then add saturated NaHCO3 to wash until neutral. The reaction solution was separated into layers, and the organic layer was dried with anhydrous MgSO4, filtered, and pressure evaporated to remove toluene. It was cooled to obtain 14.5 g of reddish-brown crude product, which was recrystallized with 40 mL of absolute ethanol to obtain 12.61 g of white crystals, with a yield of 81.5%, m.p. 81~82 ℃
Method 2: A method for preparing avobenzone, the method includes the following steps:
Step 1), add p-tert-butylbenzaldehyde, an alkaline catalyst and the first solvent to the condensation reaction kettle, optionally raise the temperature, add p-methoxyacetophenone to the system, and perform the condensation reaction;
Step 2), adjust step 1 to get the bodyThe pH value of the system is optionally cooled, filtered, and the filter cake obtained by washing is washed to obtain the condensation product 3-((4-tert-butyl)phenyl)-1-(4-methoxyphenyl)- 2-propen-1-one;
Step 3), put the condensate obtained in step 2, the second solvent and the catalyst into the oxidation reaction kettle, add the oxidant to the system, and perform the oxidation reaction;
Step 4), add sodium sulfite solution to the system prepared in step 3, wash, and remove the second solvent to obtain a crude product, which may optionally be purified to obtain avobenzone.

Method 3: A synthesis method of avobenzone, including the following steps:
1), the alkylation reaction uses toluene and chlorotert-butane as raw materials, aluminum trichloride as the catalyst, the reaction temperature is 23.5°C, the reaction time is 85 minutes, and p-tert-butyltoluene is obtained; preferably, The molar ratio of tert-butane chloride to toluene is 1:1.6;
2). Use potassium permanganate as the oxidant in the oxidation reaction to oxidize p-tert-butyltoluene obtained in step 1) into p-tert-butylbenzoic acid; the reaction temperature is 85°C and the reaction time is 8.5h. The molar ratio of p-tert-butyltoluene and potassium permanganate is 1:3, and the potassium permanganate oxidant is added in 5 to 8 times. Preferably, 3 mole parts of potassium permanganate oxidant are added into the reaction system in 6 times, with 0.9 mole parts being added for the first time, 0.6 mole parts being added for the second time, and 0.5 mole parts being added for the third and fourth times. The final input was 0.3 mole part, and the final input was 0.2 mole part. The final yield reached 83.1% and the purity was 98.6%.
3) In the esterification reaction, p-tert-butylbenzoic acid and methanol are used as raw materials, and the reaction is carried out in two stages; in the first stage, the molar ratio of p-tert-butylbenzoic acid to methanol is 1:3.5, and the reaction time 3h, distillation after the reaction is completed, and the water and methanol are separated; in the second stage, 3.5 mole parts of methanol are added, and the reaction time is 5h; distillation is carried out after the reaction is completed, and the water and methanol are separated; preferably, methylsulfonic acid is used as the catalyst, The molar ratio of p-tert-butylbenzoic acid to methanol was 1:7, and the reaction was refluxed for 8 hours.
4), the acylation reaction uses phenylethyl ether and acetic anhydride as raw materials, phosphotungstic acid as the catalyst, the molar ratio of anisole and acetic anhydride is 1:1.5, the reaction time is 4h, and the reaction temperature is 115°C;
5) In the condensation reaction, p-tert-butyl benzoic acid methyl ester and p-methoxyacetophenone are used as raw materials, sodium amide is used as the catalyst, xylene is the solvent, p-methoxyacetophenone and p-tert-tert. The molar ratio of butylmethylbenzoate is 1:1.4, the reaction temperature is 100°C, the reaction time is 5h, and avobenzone is finally obtained. The preferred reaction temperature is 95°C, and the molar ratio of p-methoxyacetophenone and p-tert-butyl methyl benzoate is 1:1.4.
Main reference materials
[1] Synthesis of UV absorber avobenzone
[2] CN201811032122.3 Application of avobenzone in the preparation of anti-tumor drugs
[3] CN201510282376.0 A synthesis method of avobenzone
[4] CN201510472432.7 A method for preparing avobenzone

 微信扫一扫打赏
微信扫一扫打赏

