Background and overview[1][2]
2,3-Difluorotoluene can be used as an intermediate for pharmaceutical and chemical synthesis. If 2,3-difluorotoluene is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if there is eye contact , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1-3]
2,3-Difluorotoluene can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its application are as follows:
1. Continuous oxidation of 2,3-difluorotoluene to prepare 2,3-difluorobenzaldehyde. 2,3-Difluorobenzaldehyde, molecular formula: C7H4F2O, molecular weight: 142.1, colorless to light yellow liquid . The boiling point is 64~65℃, the density is 1.301g/ml, and the flash point is 58℃. It is an intermediate for medicines, pesticides and liquid crystal materials. There is research and development of a method for continuously oxidizing 2,3-difluorotoluene to prepare 2,3-difluorobenzaldehyde using a tubular reactor with a special structure. The method is carried out according to the following steps: (1) First, at room temperature, The substrate 2,3-difluorotoluene and the carboxylic acid solvent are stirred and mixed at a volume ratio of 1:1. The oxidant and the carboxylic acid solvent are evenly mixed at a volume ratio of 1:1. Then the metal complex is mixed and poured into 2,3 -Difluorotoluene-carboxylic acid solution, pour the sodium salt into the hydrogen peroxide-carboxylic acid solution; through the required reaction time, calculate the different flow rates of the two materials, and continuously pump them into the tubular reactor through metering pumps After preheating and mixing, it enters the reaction zone for reaction. The reaction temperature is controlled by the external circulation heat exchange system; (2) The molar ratio of the reaction materials is controlled by adjusting the flow rate and weight, and the inner diameter of the tube of the tubular reactor is changed by 0.5 ~15mm, volume 25~750ml to control the residence time of the material mixing reaction from 60 to 2000s; after the reaction is completed, the product flows out from the end of the reactor into the collection tank, the product is distilled and separated, and the unreacted 2,3-difluorotoluene is recycled reaction, the product 2,3-difluorobenzaldehyde is collected after distillation and purification, in which the yield of the target product 2,3-difluorobenzaldehyde can reach 5% to 40%. This method has a short reaction time, high production efficiency, greatly optimized mass transfer and heat transfer, and a more stable and controllable reaction process, achieving stable and controllable continuous oxidation of 2,3-difluorotoluene and reducing the generation of by-products. Through the enhancement of mass and heat transfer processes and process optimization, the effective utilization of reaction materials is improved, the usage of oxidants and catalysts is further reduced, and the use of cocatalysts is avoided during the reaction process, thereby effectively saving production costs and improving existing industrialization processes. production method.

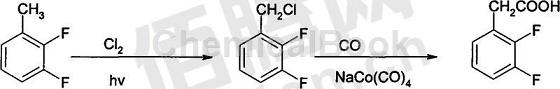
2. Preparation of 2,3-difluorophenylacetic acid. The process route is to use 2,3-difluorotoluene as the starting material, obtain 2,3-difluorobenzyl halide through photohalogenation, and then directly generate 2,3-difluorobenzyl halide from 2,3-difluorobenzyl halide through a carbonylation reaction. Difluorophenyl acetic acid. In the photohalogenation reaction, chlorine or bromine is used as the halogenating agent, and the solvent can be carbon tetrachloride or no solvent and react directly under an ultraviolet light source. 2,3-Difluorobenzyl chloride and carbon monoxide undergo a carbonylation reaction under the action of a catalyst to directly generate 2,3-difluorophenyl acetic acid. The route has fewer reaction steps, mild reaction conditions, high yield, low cost, good safety, and is easy for industrial production. It is an effective industrial green process route to avoid serious safety hazards and pollution. The development of this process route lays a good foundation for the better development of color liquid crystal materials with fluorine-containing naphthol bodies as the skeleton, and has important strategic significance for the development of localization of this series of downstream products.
3. 2,3-Difluorotoluene is used as an anti-overcharge additive for lithium-ion batteries. Lithium-ion batteries are widely used in various fields, and their safety issues have become the focus of people’s attention. Since the safety performance of ternary materials at high voltage is poor, when the lithium-ion battery is overcharged, the voltage of the battery increases with polarization. Large and rapid rise will cause irreversible changes in the structure of the positive active material and oxidative decomposition of the electrolyte, thereby generating a large amount of gas and heat, causing the battery to have safety hazards such as combustion and explosion. Among the safety additives studied in the early stage, toluene is used as an anti-overcharge additive. When the battery is charged to 4.5V, a polymerization reaction will occur, reducing the charging current and achieving the effect of preventing overcharge. And in the 4.3V high-voltage battery 50 times During the cycle, its capacity decreases rapidly. The reason is that when the 4.3V high-voltage battery is fully charged, toluene has begun to decompose, causing the internal resistance of the battery to increase, which is not suitable for high-voltage batteries. In order to solve this problem, 2,3-difluorotoluene is selected as the lithium battery. As an anti-overcharge additive for ion battery electrolyte, 2,3-difluorotoluene with strong electronegative fluorine atoms is introduced into toluene. Compared with toluene, it reduces HOMO and LUMO energy, thereby increasing the initial oxidation potential. 0.35V; when the electrolyte containing 2,3-difluorotoluene is overcharged to 4.85V in a high-voltage ternary cathode material lithium-ion battery, an electropolymerization reaction occurs, forming a fluoropolymer film with high impedance characteristics, which is effective The charging current is limited to prevent overcharging; at the same time, 2,3-difluorotoluene has good room temperature cycle in the 4.4V high-voltage ternary material battery
Main reference materials
[1] CN201610972019.1 A method for preparing 2,3-difluorobenzaldehyde by continuous oxidation of 2,3-difluorotoluene.�
[2] CN200910096369.62, preparation method for the synthesis of 3-difluorophenylacetic acid
[3] Research on the use of 2,3-difluorotoluene as an anti-overcharge additive for lithium-ion batteries

 微信扫一扫打赏
微信扫一扫打赏

