Background and overview[1][2]
4-Methoxyphenyl magnesium bromide can be used as a pharmaceutical and chemical synthesis intermediate. If 4-methoxyphenylmagnesium bromide is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1][2]
4-Methoxyphenyl magnesium bromide can be used as a pharmaceutical and chemical synthesis intermediate. For example, the following compounds are synthesized:
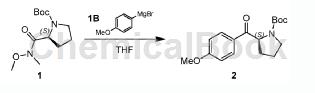
The specific steps are: cool the THF solution of 4-methoxyphenylmagnesium bromide IB (98.18g, 464.55mmol) to 0℃, and then add compound 1 (40.00 g, 154.85 mmol) in THF (150.00 mL). The mixture was stirred at 25°C under N2 atmosphere for 2 hours. TLC showed complete consumption of compound 1 and the formation of a new spot. The reaction was quenched with saturated aqueous NaHCO solution. Extract with EtOAc (100mL×3). Wash the combined organic phases with brine (200 mL × 1), dry over anhydrous Na2SO4, filter and concentrate in vacuo. The residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate = 100:1 to 20:1). Compound 2 (28.00 g, crude product) was obtained as a white solid. 1H NMR (400MHz, CDCl3): δ= 8.00-7.88 (m, 2H), 6.99-6.89 (m, 2H), 5.35-5.09 (m, 1H), 3.90-3.84 (m, 3H), 3.72-3.38 ( m, 2H), 2.36-1.80 (m, 4H), 1.52-1.13 (m, 9H). TLC (petroleum ether: ethyl acetate = 1:1) Rf = 0.43.
In addition, 4-methoxyphenylmagnesium bromide can also synthesize the following compounds:
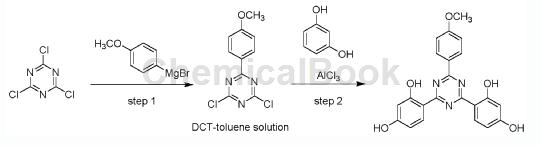
Step 1: Synthesis of 2,4-dichloro-6-(4-methoxyphenyl)-1,3,5-triazine toluene solution
In a 500 mL four-neck flask equipped with argon, a mechanical stirrer, a reflux condenser, a thermometer and an addition funnel, suspend 11.90 g of magnesium chips (0.488 mol, 1.20 eq) and a few grains of iodine in 130 mL of dry Medium THF. Under dry protective gas (nitrogen or argon), add a 5% solution of 92.2 g 4-methoxyphenylmagnesium bromide (61.7 mL, 0.488 mol, 1.20 equiv) in 116 mL anhydrous THF. An exothermic reaction indicates the onset of the Grignard reaction, then the remaining 4-bromoanisole solution is slowly added. After the magnesium chips are completely added and dissolved, the jacketed reactor is heated to 70°C and stirred for 2-4 hours until all the magnesium chips are dissolved and complete conversion of 4-bromobenzyl alcohol is observed. The resulting Grignard reagent solution was then added dropwise to a suspension of 74.9 g cyanuric chloride (0.407 mol, 1.0 equivalent) in 103 mL anhydrous THF at 0-5°C. After the addition is complete, the reactor is warmed to 25°C and the reaction mixture is stirred for an additional 30 minutes. Then, a vacuum (~300 mbar) was applied and the reactor was heated to 50°C to distill out approximately 140 ml of THF. Subsequently, 700 mL of toluene was added continuously while the THF/toluene mixture was distilled off. Then slowly add 500 mL 1N HCl. The phases were separated to obtain 707g of 2,4-dichloro-6-(4-methoxyphenyl)-1,3,5- A solution of triazine (DCT/M) in toluene (approximately 14.7 wt% DCT/M).
Step 2: Synthesis of 4,4′-[6-(4-methoxyphenyl)-1,3,5-triazine-2,4-dienyl-bis-1,3-phenylenedi Alcohol
Into a 1.5L glass reactor with an argon inlet, add 94 g of resorcinol (0.857 mol, 2.1 equivalents relative to DCT/M) and 3.2 mol eq. Benzonitrile (relative to DCT/M) was added to a solution of 707 g of the corresponding DCT/M in toluene. The resulting solution was heated to 60°C. Then 112g AlCl3 (0.842 mol, 2.07 equivalents relative to DCT/M) was added in portions. After the addition is complete, the reaction mixture is maintained at 60°C approximately 20°C. Stop heating after 4 hours of complete conversion. Then 55mL 1N HCl (55mmol) was added dropwise, followed by 150mL toluene, 165mL 1N HCl (165mmol) and 150mL water. The resulting suspension was filtered, followed by washing with 350 mL of toluene and 1000 mL of water. The filter cake was sucked dry and then dried under vacuum (~100 mbar) at 60°C overnight to give the specified yield of 4,4′-[6-(4-methoxyphenyl) -1,3,5-Triazine-2,4-diyl]-bis-1,3-benzenediol (MTB).
Preparation[1][2]
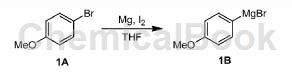
A mixture of Mg (13.49g, 554.99mmol) and I2 (50.00mg, 197.00μmol) in THF (260mL) was degassed and purged with N 3 times, then compound 1A (86.50g, 86.50g, 462.49mmol) in THF (200mL). The mixture was stirred at 25°C under N2 atmosphere for 1 hour. The color of the mixture changes. The crude product compound 1B was used in the next step without further purification (97.74 g, crude).
Main reference materials
[1] WO2018237194. COMPOUNDS, COMPOSITIONS AND METHODS FOR SYNTHESIS
[2] WO2016184764. PROCESS FOR THE PREPARATION OF TRIAZINES

 微信扫一扫打赏
微信扫一扫打赏

